H.pylori Test Kit
(2024年02月27日)https://www.medical-ivd.com/products/h-pylori-test-kit.html
H.pylori infects more than half the people in the world. The prevalence of the infection varies among countries and among different groups within the same country. The prevalence rate in the United State suggests an incidence of infection of 2%. The lifetime prevalence of peptic ulcer disease is about 12% in men and 9% in women.
Studies have found that more than 90% of patients with duodenal ulcer and 80% of patients with gastric ulcer are infected with H. Pylori Antigen rapid test IVD kit detects the presence of H.pylori antigens in stool specimens. Expected values for any given population should be determined for each laboratory. The positivity rate of any given laboratory may vary depending on geographic location, ethnic group, and living environment.
H.pylori Test Kit Features
This h pylori medikament test kit is for qualitative detection of H. pylori antigen in stool sample and dose not indicate the quantity of the antigens. This h pylori test kit is for in vitro diagnostics use only. This h pylori test kit result should be used only to evaluate with patient with signs and symptoms of gastrointestinal disease. A definitive clinical diagnosis should only be made by the physician after all clinical and laboratory finding have been evaluated.
H.pylori Test Kit Priciples
H. pylori Antigen Rapid Test Kit simplified as h pylori kit, is a sandwich solid phase immunochromatographic assay. To perform the test, an aliquot of diluted stool sample is added to the sample well of the test cassette. The sample flows through a label pad containing H. pylori antibody coupled to redcolored colloidal gold. If the sample contains H. pylori antigens, the antigen will bind to the antibody coated on the colloidal gold particles to form antigen-antibody-gold complexes. These complexes move on the nitrocellulose membrane by capillary action toward the test line region on which H. pylori specific antibodies are immobilized.
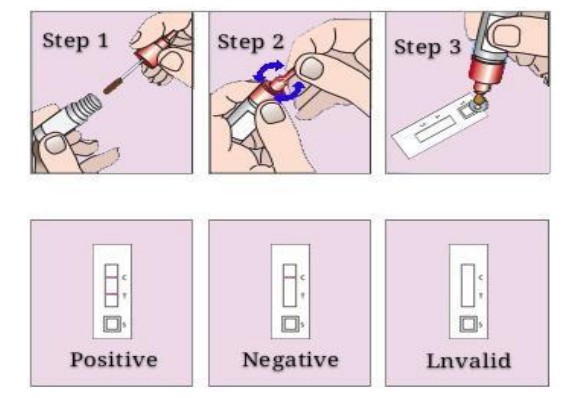
How to Use H.pylori Kit
1. Drop two drops of reagent ⅰ into the central hole of the reaction plate and wait for it to penetrate completely;
2. Drop 100µl of serum into the wells of the reaction plate until it is completely infiltrated;
3. Add three drops of Reagent II dropwise to the wells of the reaction plate and wait for it to penetrate completely;
4. Infiltrate three drops of reagent ⅰ into the wells of the reaction plate and wait for complete infiltration.
5. Serum samples cannot be hemolyzed and should be stored in fresh serum or at 2°C to 8°C for no more than a week.
6. Hyperlipidemia serum cannot be used.
Our core competence lies in our ability to provide needed reagents, IVD raw materials, and custom IVD test kits services to in vitro diagnostic solutions investigators and researchers. We excel in being a trusted source for our clients around the globe. Contact us for more IVD products list details.
- «前のできごと |
- 次のできごと»
- このできごとのURL:






コメント