IVD Test Kit
(2024年02月27日)https://www.medical-ivd.com/ivd-test-kit/
We specialize in providing reliable in vitro diagnostic rapid test kits for detecting various infectious diseases. Our product offerings include colloidal gold antibody tests, ebola test kits, hepatitis C test kits, dengue test kits, H.pylori test kits, and malaria test kit for sale. As experienced diagnostic kit manufacturers, our dedicated team of professionals works tirelessly in research and development, manufacturing, sales and marketing, and customer service to ensure the superior quality of production and placement of in vitro diagnostic test kits, rapid test IVD kits etc. We also offer reasonable IVD test kits price, so that everyone can access the diagnostic tools they need to monitor and maintain their health.
In Vitro Diagnostic Test Kits Types
In Vitro Diagnostic Test Kits have revolutionized the way healthcare providers diagnose and treat different conditions. They allow for rapid and accurate testing in a wide range of settings, including hospitals, clinics, labs, and even at home. There are many different types of diagnostic kits tailored to detect specific conditions, including infectious diseases like COVID-19, sexually transmitted infections, allergies, pregnancy, cancer, and autoimmune disorders. The following is the introduction to the different types of in vitro diagnostic kits provided by us.
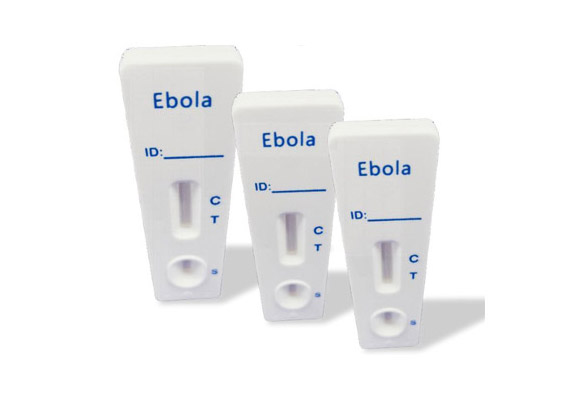
Ebola Test Kit
The in vitro diagnostic rapid test for Ebola (Colloidal gold method) is designed to quickly and accurately detect the presence of Ebola antigens from nose swabs. This test is intended to be used as a supportive diagnostic tool, providing healthcare professionals with crucial information to aid in the diagnosis of Ebola cases. With its efficient and reliable results, the in vitro diagnostic rapid test for Ebola plays an important role in enabling healthcare professionals to make informed decisions and provide timely care for their patients.
HCV Test Kit
The Hepatitis C Virus (HCV) is a small, enveloped, positive-sense RNA virus, responsible for a significant amount of parenterally-transmitted non-A, non-B hepatitis cases. By using advanced in vitro diagnostic kits for detecting HCV, healthcare providers can quickly and accurately diagnose this infectious disease, giving them important information to provide proper care and treatment to patients.
Dengue Test Kit
Dengue virus is an enveloped, single-stranded, positive-sense RNA virus that comprises four related but distinct serotypes (Den 1, 2, 3, and 4). The virus is transmitted by mosquitoes of the daytime-biting Stegomyia family. Accurate and timely diagnosis of dengue fever is crucial for effective treatment, and in vitro diagnostic kits designed to detect dengue-specific biomarkers enable healthcare professionals to provide more reliable and efficient diagnoses.
Malaria Test Kit
Malaria is a serious parasitic disease characterized by fever, chills, and anemia and is caused by a parasite that is transmitted from one human to another by the bite of infected Anopheles mosquitoes. In vitro diagnostic rapid test represents an essential tool in identifying specific biomarkers in the blood to determine the presence of malaria parasites, harnessing advanced technological innovations to provide quick and reliable results.
H.pylori Test Kit
H.pylori infects more than half the people in the world. The prevalence of the infection varies among countries and among different groups within the same country. In vitro diagnostic test kits have played an important role in combating H. pylori infection, leading the way to early detection and better management of this debilitating condition.
One Step Rapid Diagnostic Test for in Vitro Diagnostic Use Only
Rapid test IVD device can provide rapid detection of pathogens and other molecules in biological samples. Rapid test in vitro diagnostic test kits can produce results within minutes of exposing the sample, thereby allowing for on-the-spot diagnosis and treatment. This speed is of the utmost importance during emergency situations and also highly convenient in busy clinical settings.
After the occurrence of Corona Virus Disease 2019, how to quickly and accurately screen and diagnose patients with new coronary pneumonia has become the top priority of epidemic prevention and control. Choose our in vitro diagnostic devices!
Medikament-produced In Vitro Diagnostic Test Kits Advantages
Being a professional IVD test kits supplier, we advanced engineering techniques and a powerful design team to help you utilize our unique retail fast in vitro diagnostic test kits. We get the full ability to provide you high-quality IVD medical device with simple-to-use formats for you and your customers.
In Vitro Diagnostic Test Meaning
IVD (In Vitro Diagnosis) refers to products and services outside the human body that obtain clinical diagnostic information by testing human samples (blood, body fluids, tissues, etc.) to determine diseases or body functions.
After the occurrence of Corona Virus Disease 2019, how to quickly and accurately screen and diagnose patients with new coronary pneumonia has become the top priority of epidemic prevention and control.
An inspection kit is a common in vitro diagnostic medical device, also known as IVD kit, which refers to a kit for inspecting a specific item, usually used in in-vitro diagnostic tests. The commodity form is a packaging box that contains all the reagents of the item. For example, ELISA surface antigen detection kit includes coating plate, yin-yang control, enzyme reagent, plate washing solution, chromogenic solution, termination solution, etc. The test reagent management system is a professional IVD diagnostic reagent management software, which is specially developed for the efficient and accurate management of reagents in the laboratory. In vitro diagnostic rapid test device is applicable to the laboratory departments of hospitals, blood centers, and CDC.
Rapid Test Kit Colloidal Gold
The in vitro diagnostic kits are simple and fast to operate, no ceremony is required, and the detection time only takes 5-15 minutes. It is suitable for rapid on-site detection in grassroots hospitals, disease control institutions, airports, customs, etc., and fully serves the needs of epidemic prevention and control.
The product is lightweight, portable, and suitable for places where a large-scale investment is in dense crowds
The rapid test IVD kits sold by our company, including Ebola Test Kit, HCV Test Kit, Dengue Test Kit, Malaria Test Kit, H.pylori Test Kit, can be used in various scenarios.
Colloidal Gold Antigen Test Instructions
Colloidal gold is a commonly used labeling technique. It is a new type of immunolabeling technique that uses colloidal gold as a tracer to be applied to antigen and antibody. It has its unique advantages. It has been widely used in various biological researches in recent years. Almost all immunoblotting techniques used in the clinic use its markers. At the same time, it can be used in flow cytometry, electron microscopy, immunology, molecular biology and even biochips.
Ebola Test Kit is a Colloidal Gold Antigen test product recommended by our company, which can quickly and accurately produce results.
Colloidal Gold Antigen Test Accuracy
Mainly depends on whether the sensitivity and specificity of the raw materials such as monoclonal antibodies, polyclonal antibodies, antigens, haptens, protein chimeras, and colloidal gold can meet the highest standards
It also depends on the protein purification method, antibody subtype, protein purity and preservation solution.
Selecting the right in vitro diagnostic test kits
1. Clinical need: The first step in selecting an in vitro diagnostic rapid test kit is to determine the clinical need for the test, such as diagnosing a specific condition, monitoring treatment efficacy, or detecting disease progression. The test kit should be designed to detect the biomarkers related to the specific clinical need, and the test results must be clinically relevant.
2. Analytical performance: In vitro diagnostic test kits are classified based on their analytical and clinical performance characteristics. The analytical performance characteristics include sensitivity, specificity, accuracy, precision, and reproducibility. These parameters should be considered when selecting ivd test kits, as they determine the reliability and accuracy of the test results.
3. Regulatory compliance: In vitro diagnostic kits must comply with regulatory guidelines set by the Food and Drug Administration (FDA) and other regulatory agencies. The test kit must be approved or cleared by the regulatory authority before it can be used clinically.
4. Complexity and ease of use: The complexity of the IVD kit and its ease of use are important factors to consider, especially in the case of point-of-care testing (POCT). The in vitro diagnostic rapid test kit should be easy to use and require minimal technical expertise to perform the test and interpret the results.
5.Cost and availability: The IVD test kits price and availability are factors that need to be considered when selecting the test kit. The test kit should be affordable and readily available in the market.
Q
What is the in vitro diagnostic test meaning?
A
An inspection kit is a common in vitro diagnostic medical device, also known as IVD kit, which refers to a kit for inspecting a specific item, usually used in in-vitro diagnostic tests. The commodity form is a packaging box that contains all the reagents of the item. For example, ELISA surface antigen detection kit includes coating plate, yin-yang control, enzyme reagent, plate washing solution, chromogenic solution, termination solution, etc. The test reagent management system is a professional IVD diagnostic reagent management software, which is specially developed for the efficient and accurate management of reagents in the laboratory. In vitro diagnostic device is applicable to the laboratory departments of hospitals, blood centers, and CDC.
Q
What is the difference between in vitro diagnostic test kits and over-the-counter (OTC) home test kits?
A
In vitro diagnostic test kits are usually designed for clinical laboratories and require technical expertise to perform the test and interpret the results. On the other hand, OTC home test kits are designed for self-use and are available without a prescription. OTC home test kits are often simpler to use and provide rapid results.
Q
How are in vitro diagnostic kits results reported?
A
In vitro diagnostic test kit results are reported in different formats and units, depending on the test kit and the biomolecule detected. The results can be qualitative or quantitative, and they may be reported as positive or negative, or in numerical values.
Q
How long does it take to perform an in vitro diagnostic rapid test?
A
The time required to perform an in vitro diagnostic test depends on the complexity of the test kit and the type of biomolecule detected. Some tests can provide results within a few minutes, while others may take several hours or days to complete.
Q
Can in vitro diagnostic test kits produce false-positive or false-negative results?
A
Yes, in vitro diagnostic test kits can produce false-positive or false-negative results, depending on various factors. False-positive results occur when the test kit detects the biomolecule in the absence of the disease or condition, while false-negative results occur when the test kit fails to detect the biomolecule in the presence of the disease or condition.
Q
What are the regulatory requirements for in vitro diagnostic test kits?
A
In vitro diagnostic test kits are subject to regulatory oversight by the Food and Drug Administration (FDA) and other regulatory agencies. The test kits must be approved or cleared by the regulatory authority before they can be used for clinical diagnosis and management of diseases.
Q
What is the detection principle of colloidal gold rapid detection card?
A
Colloidal gold rapid detection card is a type of colloidal gold antigen test developed by colloidal gold immunochromatography technology. This technology is a fast and simple immunological detection technology established on the basis of immune permeation technology in the early 1990s. There are the double antibody sandwich method, double antigen sandwich method, small molecule competition method, and so on.
With the continuous expansion of the IVD market and in vitro diagnostic solutions industry, we not only provide high-quality IVD raw materials such as CMIT/MIT Preservation, IVD preservatives, monoclonal antibodies, and antigens for in vitro diagnostics industry but also provide IVD test kits like Ebola test kit, HCV test kits, dengue test kit, etc.
- «前のできごと |
- 次のできごと»
- このできごとのURL:






コメント