GMP Compliance & Certification
(2024年02月01日)https://www.chinesepeptideco.com/gmp-compliance-certification.html
CPC manufactures peptide active pharmaceutical ingredients (APIs) in accordance with FDA/EU cGMP guidelines, which include:
Code of Federal Regulations Part 210/211 (21 CFR 210/211)
ICH Q7 (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients Q7)
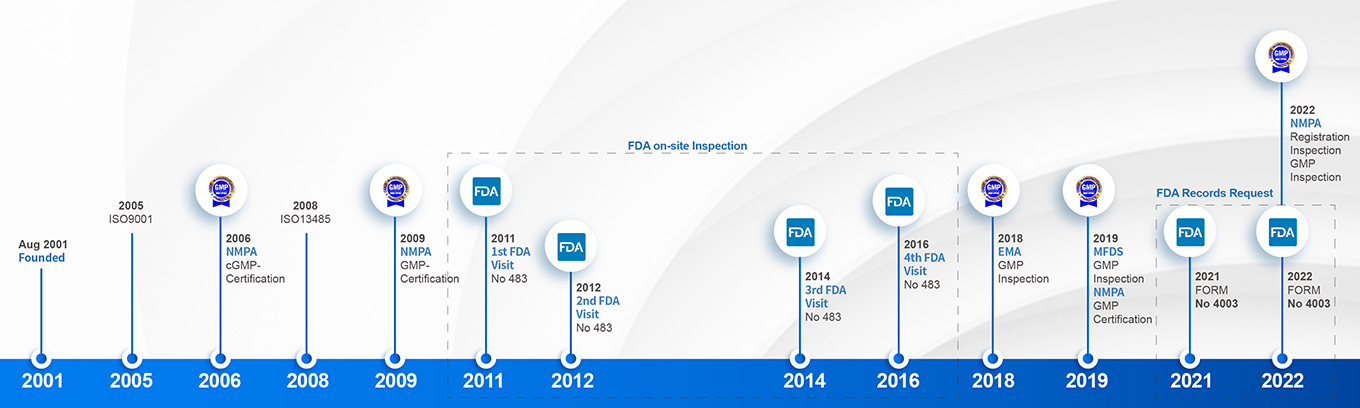
Check our audition and Inspection History here:
GMP regulatory compliance History Timeliness
CGMP Compliance
The CPC Quality department is committed to ensuring complete adherence to CGMP standards at every stage of production. Additionally, they rigorously uphold testing and documentation protocols in alignment with the most stringent FDA stipulations.
In-process, release and environmental control testing performed
Standard release testing performed in-house
Audited contract laboratories available for additional testing
Method development and validation, formal method transfer
Stability studies (ICH) and forced degradation.
As one of the gmp certified supplement companies, we provide gmp manufacturing license, good manufacturing process certification, regulatory certification of gmp, fda gmp certified facility, etc. For more information, please feel free to contact us!
There are many peptide manufacturing companies, but we are one of the best choices for you.
- «前のできごと |
- 次のできごと»
- このできごとのURL:



コメント