Thrombite™ Clot Retriever Device
(2022年11月19日)https://www.zyloxtb.com/healthcare-professionals/thrombite-clot-retriever-device/
More efficient choice of recanalization
OVERVIEW
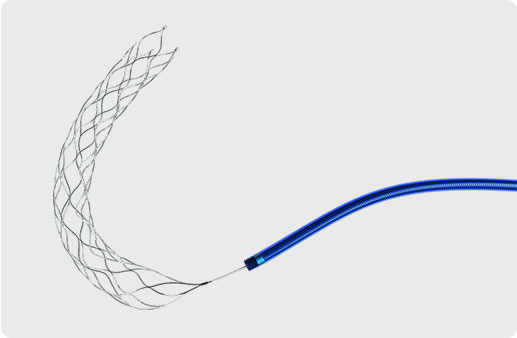
Thrombite™ Clot Removal Device
HELICAL OPEN-SIDE STRUCTURE
HIGHER ACUTE RECANALIZATION RATE
The Thrombite™ Clot Retriever Device, featuring S-shaped helical open-side structure, is designed for more efficient clot removal and optimum revascularization in a wide range of vessels.
PRODUCT FEATURES
HELICAL OPEN-SIDE STRUCTURE
enables Thrombite™mechanical retriever to efficiently entwine and clamp the clot
assures clot retention for confident removal and revascularization
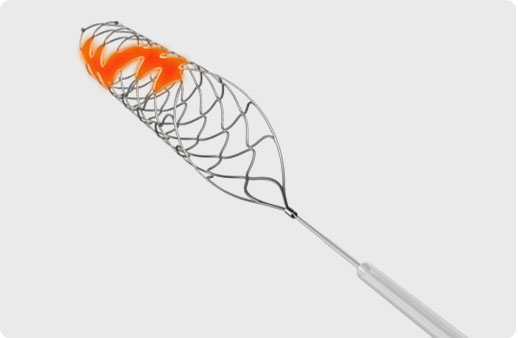
OVERLAPPED STENT AT THE HELICAL OPEN-SIDE STRUCTURE
increases contact surface with thrombus and maximizes clot integration
EXCELLENT FLEXIBILITY AND VESSEL WALL APPOSITION
optimizes clot retention during smooth navigation through the tortuous anatomies
Radiopaque markers on the distal end of the device increase device visibility
3 markers for the sizes with 3.0 and 4.0mm diameter
4 markers for the sizes with 5.0 and 6.0mm diameter
Advanced stent surface treatment process
RADIAL FORCE
High radial force is designed for clot integration at the initial stage of stent expansion
Low radial force at nominal stent diameter ensures atraumatic retrieval
How a Stent Retriever Procedure Is Performed
Stent retriever procedure is generally performed in patients with the indications of acute cerebral infarction within six hours. After femoral artery puncture, the catheter is inserted after sheath puncture, and DSA is performed along the catheter to the brain to find the position of blocked vascular. Make the stent retriever reached the position and passing through the clot. Then release to capture the clot. After that, the stent retriever will be extracted along with the clot and finally leave your body.
ORDERING INFORMATION OF CLOT RETRIEVAL DEVICE
STENT RETRIEVAL DEVICE STRUCTURE
Thrombite™Product codeDiameter D(mm)Recommended Vessel Diameter(mm)
Min. Max.Recommende Microcatheter ID (inch)Working Length A(mm)Stent Length B(mm)System Length(mm)
3MMCRD-3-1531.53.00.2115271900
CRD-3-2031.53.00.2120321900
CRD-3-2531.53.00.2125371900
CRD-3-3031.53.00.2130421900
4MMCRD-4-1542.04.00.2115271900
CRD-4-2042.04.00.2120321900
CRD-4-2542.04.00.2125371900
CRD-4-3042.04.00.2130421900
5MMCRD-5-1552.55.00.02715271900
CRD-5-2052.55.00.02720321900
CRD-5-2552.55.00.02725371900
CRD-5-3052.55.00.02730421900
6MMCRD-6-1563.05.50.02715271900
CRD-6-2063.05.50.02720321900
CRD-6-2563.05.50.02725371900
CRD-6-3063.05.50.02730421900
INDICATIONS
Thrombite™ Clot Retriever Device
The Thrombite™ endovascular clot retrieval devices are intended to restore blood flow by removing thrombus from a large intracranial vessel in patients experiencing ischemic stroke symptom onset.
If you want to know more about blood clot retrieval devices market, please leave us a message.
Zylox-Tonbridge said that circulation of its H shares will contribute to enhance their liquidity and the Company's circulation market capitalization, thereby attracting a greater number of foreign and local investors. In the long run, it will also help improve the Company’s governance structure and better stimulate the power for its development, as well as achieve long-term steady growth and investment returns. Zylox-Tonbridge Medical will continue to improve its capital strength so as to better meet the needs of high-quality development of the Company.
- このできごとのURL:


コメント