Swimming Pool
(2020年06月10日)http://www.seawaterchlorination.com/application/swimming-pool.html
Description of swimming pool chlorination systems
The Milestone Chlorination Technologies designed brine electro chlorination system is one of the best to support the swimming pool engineering company to give one stop solution to client.
Unlike salt chlorinator or so called salt chlorination system, the system does not require salinity in the swimming pool and your swimming pool will not get too salty while swim.
As require less salt and unique design in electrolyzer, it reduces a lot cost in power consumption, therefore our promoted brine electro chlorination system has lower operating cost compare to salt chlorinator.
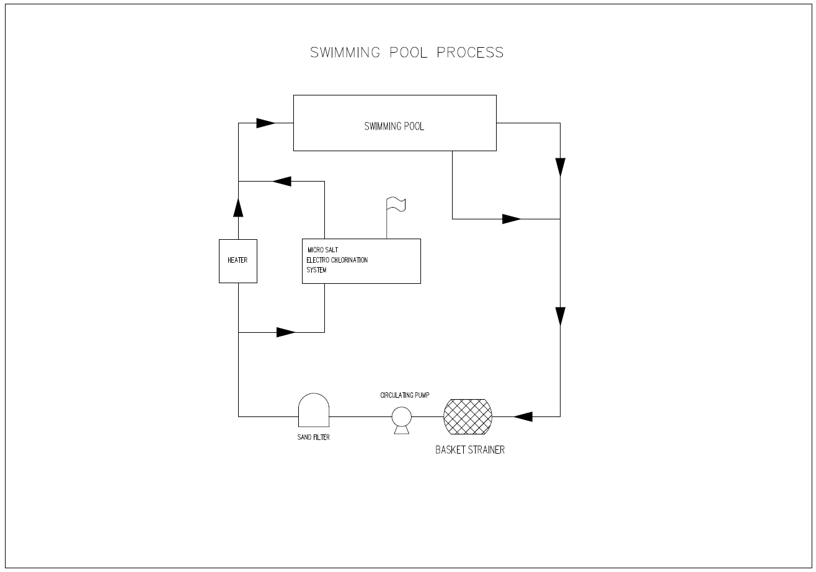
Process Flow of pool chlorination systems
Requires only Salt, Water and Electricity.
Provides the power of chlorine without the danger of storing or handling Hazardous materials.
Sodium Hypochlorite generated on-site does not degrade like commercial Sodium Hypochlorite.
The total operating cost is less than conventional Chlorination methods.
On-site generation of sodium hypochlorite allows the operator to produce only What is needed and when it is needed.
On site hypo sodium hypochlorite generation system is a disinfection alternative that small water treatment systems will find beneficial because it is cost-effective, easy to produce and eliminates potentially dangerous handling and storage problems associated with other types of disinfection practices. On-site Sodium Hypochlorite (NaOCl) generation requires only salt, water and electricity to produce sodium hypochlorite actually needed. Unlike conventional 12 to 15 percent purchased hypochlorite, which will degrade over time, Sodium Hypochlorite generated on-site will maintain its strength.
Within the Electrolyzer, the brine solution or seawater, which is a good conductor of electricity, supports a current applied between the positive and negative electrodes, thus electrolyzing the sodium chloride solution. These results in chlorine (Cl2) gas being produced at the positive electrode (anode), while sodium hydroxide (NaOH) and hydrogen (H2) gas are produced at the negative electrode (cathode). The chlorine further reacts with the hydroxide to form sodium hypochlorite (NaOCl).
This reaction can be simplified as follows:
NaCl + H2O + Electricity = NaOCl + H2
(Salt) (Water) (Sodium Hypochlorite) (Hydrogen)
The electrolytic process of electrochlorination of seawater type sodium hypochlorite generator is a process of electrochemical reaction. The only two materials needed are water and salt; no additional material is needed. The sodium hypochlorite solution produced is very pure. As this system is applied to disinfection of tap water, the design of the generator system has fully considered the characteristics of energy-saving operation, cost saving, high reliability, long service life, easy operation, etc.
- «前のできごと |
- 次のできごと»
- このできごとのURL:


コメント